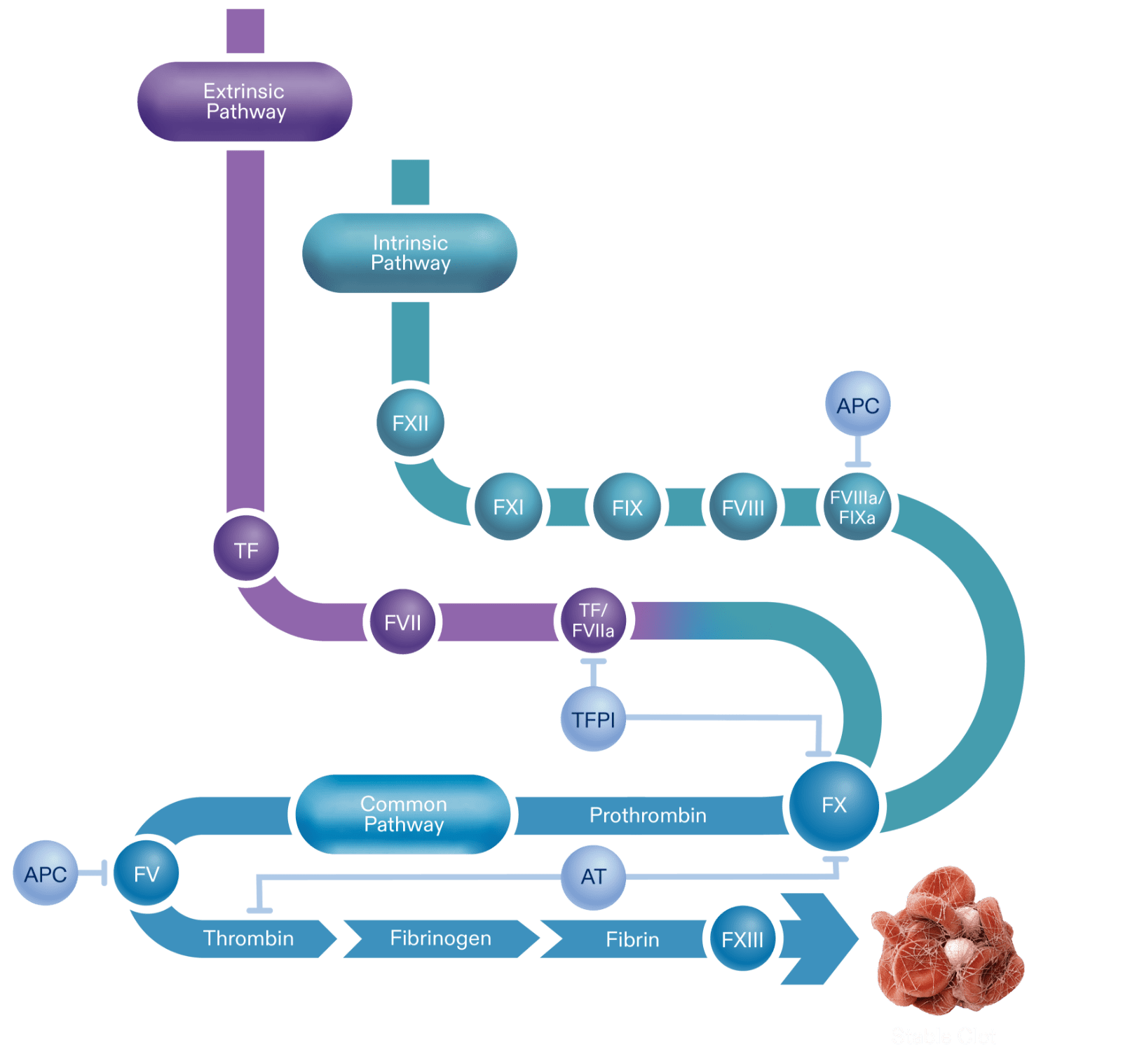
Thrombin made via the initiation phase is not sufficient to create a clot so it binds with platelets in a feedback loop. This amplification phase, or intrinsic pathway, generates a “thrombin burst.”
Following a breach in the integrity of endothelium and vasculature tissue, the hemostatic response begins with the extrinsic pathway, or initiation phase.
The intrinsic pathway starts with the activation of Factor XII which then activates Factor XI.
Activated protein C (APC) is an anticoagulant that inhibits FVIIIa and FVa, preventing further thrombin propagation.
The activated forms of Factor VIII and Factor IX bond together to form FVIIIa/FIXa, the intrinsic tenase complex. Without this, thrombin generation is limited.
The activation of Factor XI is needed to activate downstream factors: FVIII, and FIX.
Factor IX is a procoagulant. Its activity is enhanced through its interaction with FVIII.
Factor VIII is a co-factor to factor IX. It is a stabilized by von Willebrand factor in its inactive form.
Upon exposure, tissue factor (TF) initiates coagulation to prevent blood loss.
TF combines with Factor VII, a procoagulant, to form TF/FVIIa.
The TF/FVIIa complex is needed for the initial activation of Factor X. This complex is also primarily inhibited by the anticoagulant, TFPI.
Tissue factor pathway inhibitor (TFPI) is an anticoagulant that inhibits TF-FVIIa complex and FX.
The enzymatic reactions of each pathway lead to Factor X activation, a key component to thrombin generation.
Activated protein C (APC) is an anticoagulant that inhibits FVIIIa and FVa, preventing further thrombin propagation.
The activated FX eventually joins activated Factor V, initiating the conversion of prothrombin to thrombin.
Antithrombin (AT) is an anticoagulant that is able to inhibit thrombin directly, as well as FXa and other coagulation factors.
Activated FXIII cross-links fibrin fibers, stabilizing the clot.
Once FX is activated, the cascade continues via the common pathway. This is the final enzymatic stage of the cascade that generates thrombin, and subsequently fibrin to help form a stable clot. This path, or propagation phase, can be inhibited by AT, APC, and TFPI.
Prothrombin is converted to thrombin via FXa/FVa, also known as the prothombinase complex.
Thrombin is a serine protease that converts fibrinogen to fibrin. Thrombin formed in this phase is also able to support the amplification phase, by activating FXI, and cofactors FV and FVIII on the platelet surface.
Circulating fibrinogen can be converted to fibrin.
Fibrin fibers form a mesh structure to secure the clot.

A balanced amount of anticoagulants and procoagulants creates a stable clot.